Accelerated Design of Redox-Active Polymers for Metal-Free Batteries
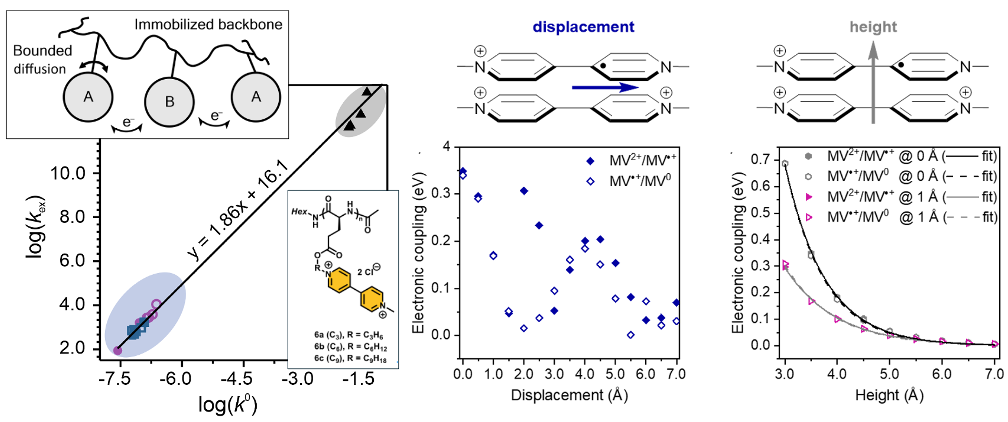
Redox-active polypeptides offer a route toward fully metal-free, bio-inspired batteries. The active groups are separated from the polypeptide backbone by a linker group, but the effect of the linker group’s length on the kinetics of the redox reaction was unknown. Viologen-containing polypeptides of linker groups with varying spacer lengths were synthesized and the electron transfer kinetics were measured. Only the longer alkyl spacer lengths of six or nine carbons allowed for the polypeptide backbone to adopt an alpha-helical conformation, which leads to a very rigid backbone.
The apparent heterogeneous and homogeneous rate constants (kex and ko) were measured using chronoamperometry. Viologen exhibited two one-electron transfer steps for which the kinetics followed a trend according to barriers associated from transfer for charged to neutral states and vice versa. Interestingly, log-log plots of kex and ko revealed a linear relationship. Analysis of the resulting slope revealed that the redox mechanism follows Marcus–Hush theory of electron transfer with diffusion limitations, or Laviron–Andrieux–Savéant theory. Galvanostatic cycling of these materials showed that the polypeptide with a spacer group of six carbons exhibited the highest capacity, having good kinetics with lower swelling.