Metal-free Aqueous Energy Storage Electrodes
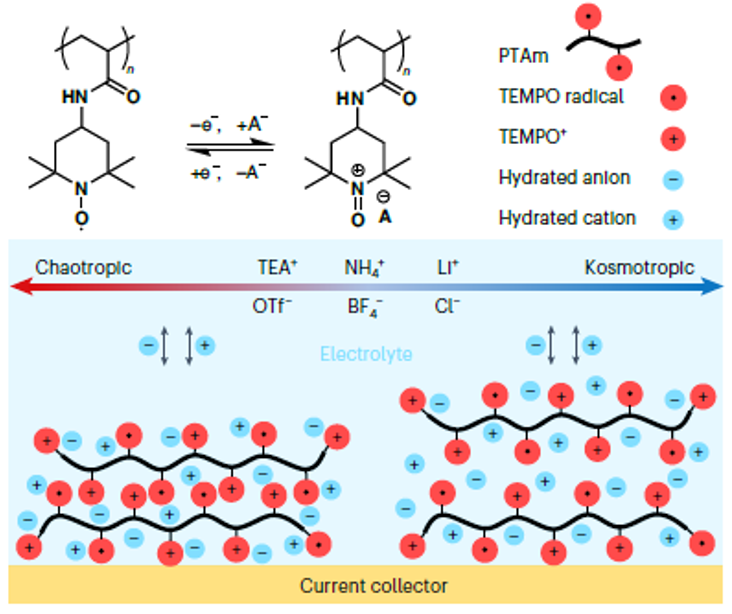
Metal-free aqueous batteries can potentially address the projected shortages of strategic metals and safety issues found in lithium-ion batteries. More specifically, redox-active non-conjugated radical polymers are promising candidates for metal-free aqueous batteries because of the polymers’ high discharge voltage and fast redox kinetics. However, little is known regarding the energy storage mechanism of these polymers in an aqueous environment. The reaction itself is complex and difficult to resolve because of the simultaneous transfer of electrons, ions and water molecules. Here, the nature of the redox reaction was demonstrated by examining aqueous electrolytes of varying chao-/kosmotropic character using electrochemical quartz crystal microbalance with dissipation monitoring at a range of timescales. Surprisingly, the capacity can vary by as much as 1,000% depending on the electrolyte, in which certain ions enable better kinetics, higher capacity, and higher cycling stability. The findings of this work will provide insights into the general electrochemistry of redox-active polymers regarding ionic transfer and diffusion often observed with batteries, sensors, actuators, and other devices and materials.