Solid-state Phthalimide-containing Polymers for All-organic Batteries
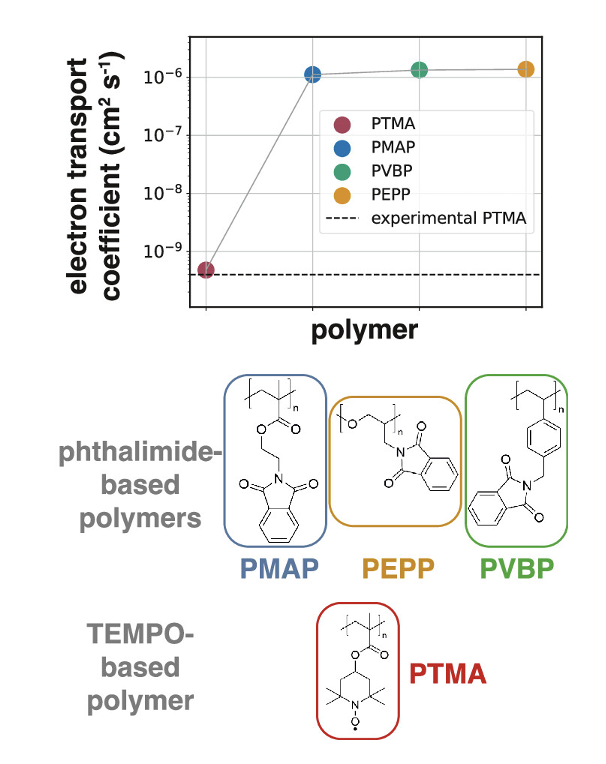
Redox-active polymers serving as the active materials in solid-state electrodes offer a promising path toward realizing all-organic batteries. While both cathodic and anodic redox-active polymers are needed, the diversity of the available anodic materials is limited. Here, solid-state structural, ionic, and electronic properties of anodic, phthalimide-containing polymers are predicted using a multiscale approach that combines atomistic molecular dynamics, electronic structure calculations, and machine learning surrogate models. By combining information from each of these scales, it is possible to bridge the gap between bottom-up molecular characteristics and macroscopic properties such as apparent diffusion coefficients of electron transport (Dapp). The impact of different polymer backbones, state of charge, and polymer swelling during battery operation were investigated. It was revealed that the state of charge significantly influences solid-state packing and the thermophysical properties of the polymers, which, in turn, affect ionic and electronic transport. A combination of molecular-level properties and condensed-phase properties determine the predicted ranking of electron transport capabilities of the polymers. Dappwas predicted for the phthalimide-based polymers and for a reference nitroxide radical-based polymer, finding a 3 orders of magnitude increase in Dapp with respect to the reference. This study underscores the promise of phthalimide-containing polymers as highly capable redox-active polymers for anodic materials in all-organic batteries, due to their exceptional predicted electron transport capabilities.