Perspective: Biologic Formulation in a Self-driving Biomaterials Lab
Biologics such as monoclonal antibodies (mAbs) and RNA therapeutics have revolutionized standard of care but present stability challenges due to their fragile structure. This is particularly true considering the demanding manufacturing, storage, distribution, and administration requirements that far exceed the otherwise stable biological environment from which they are derived.
Adam Gormley (Rutgers University)
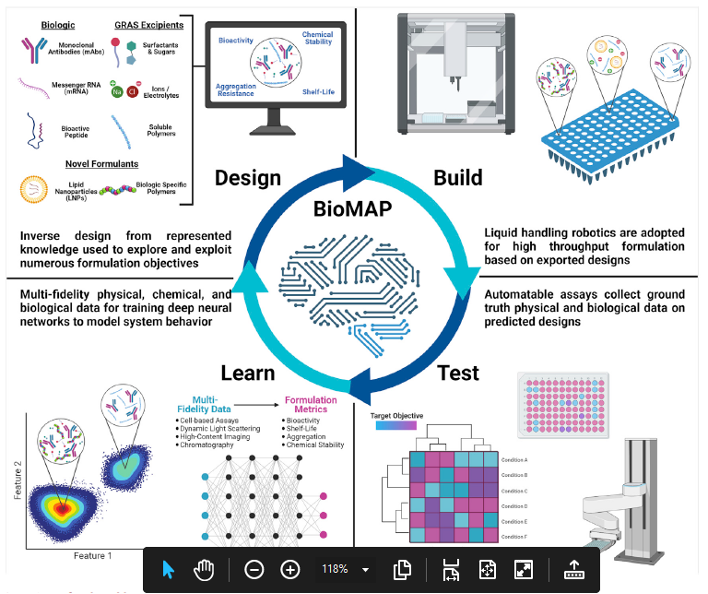
Biologics such as monoclonal antibodies (mAbs) and RNA therapeutics have revolutionized standard of care but present stability challenges due to their fragile structure. This is particularly true considering the demanding manufacturing, storage, distribution, and administration requirements that far exceed the otherwise stable biological environment from which they are derived. Therefore, the pharmaceutical industry routinely implements a suite of experiments to optimize formulations using a standard set of excipients that are known to enhance stability. While this process has been productive, the complexity of biologic-excipient interactions prevents an efficient transition to precise and tailored formulations. Recent advances in laboratory automation, high-throughput analytics, and artificial intelligence/machine learning (AI/ML) now provide a unique opportunity to fully automate the design process and provide next-generation formulations with remarkable durability. Here, a plan is put forth to develop a biomaterials acceleration platform (BioMAP) (i.e., self-driving biomaterials lab) focused initially on biologic formulation.