A New Pathway to Resilient Materials
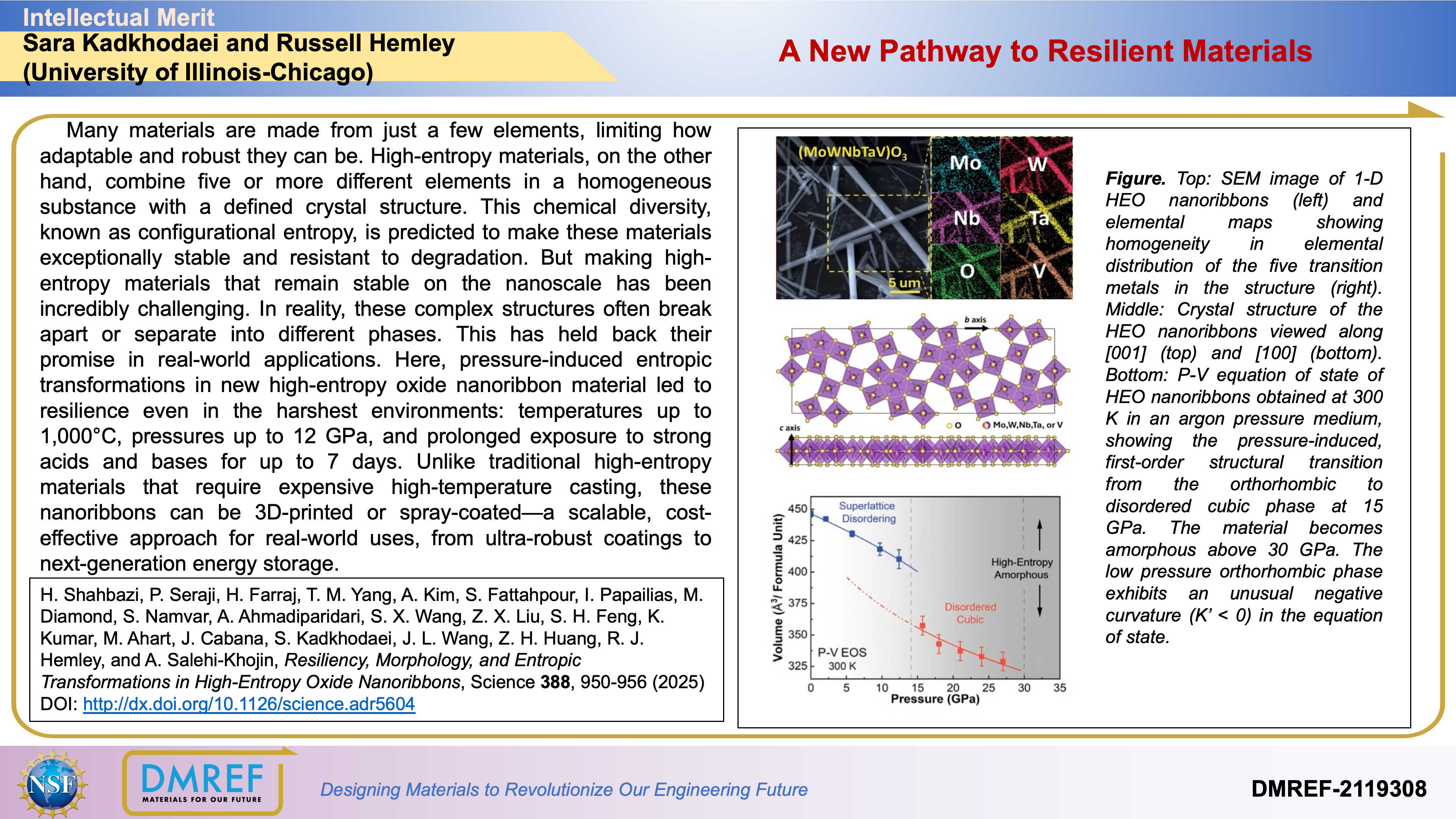
![Figure. Top: SEM image of 1-D HEO nanoribbons (left) and elemental maps showing homogeneity in elemental distribution of the five transition metals in the structure (right). Middle: Crystal structure of the HEO nanoribbons viewed along [001] (top) and [100] (bottom). Bottom: P-V equation of state of HEO nanoribbons obtained at 300 K in an argon pressure medium, showing the pressure-induced, first-order structural transition from the orthorhombic to disordered cubic phase at 15 GPa. The material becomes amorphous above 30 GPa. The low pressure orthorhombic phase exhibits an unusual negative curvature (K’ < 0) in the equation of state.](https://imagedelivery.net/qXYabcYg0JPsn0bBVWE4SA/60e5cf49-7d82-47e6-9162-764cb4b35e54/Picture1.jpg/raw)
Many materials are made from just a few elements, limiting how adaptable and robust they can be. High-entropy materials, on the other hand, combine five or more different elements in a homogeneous substance with a defined crystal structure. This chemical diversity, known as configurational entropy, is predicted to make these materials exceptionally stable and resistant to degradation. But making high-entropy materials that remain stable on the nanoscale has been incredibly challenging. In reality, thesecomplex structures often break apart or separate into different phases. This has held back their promise in real-world applications.
Here, pressure-induced entropic transformations in new high-entropy oxide nanoribbon material led to resilience even in the harshest environments: temperatures up to 1,000°C, pressures up to 12 GPa, and prolonged exposure to strong acids and bases for up to 7 days. Unlike traditional high-entropy materials that require expensive high-temperature casting, these nanoribbons can be 3D-printed or spray-coated—a scalable, cost-effective approach for real-world uses, from ultra-robust coatings to next-generation energy storage.