Selective Recovery of Platinum Group Metals
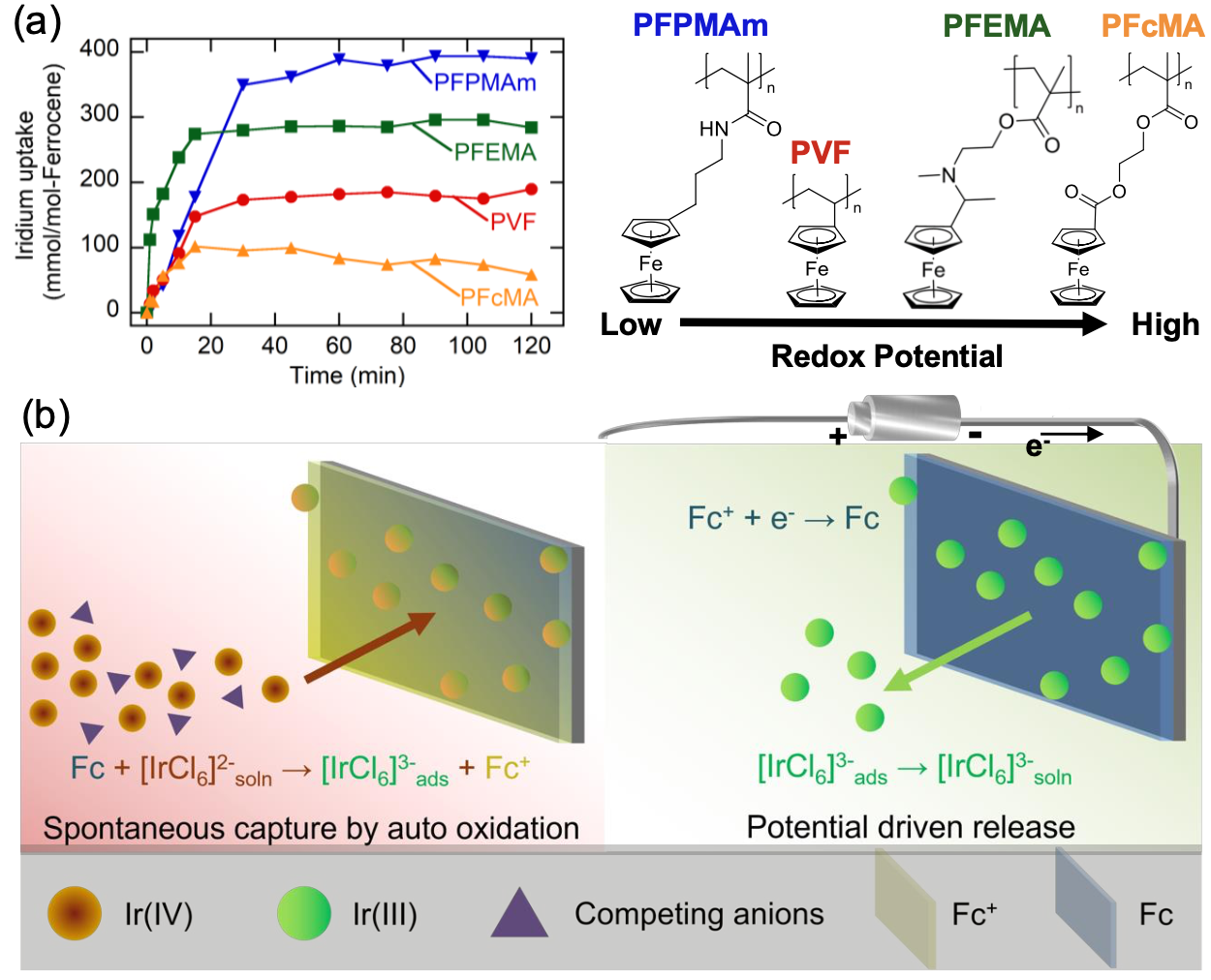
Due to the intrinsically high oxidation potential of the chloro-PGM complexes in leachate solutions, spontaneous electrochemical PGM recovery was possible without electrical or chemical input. Here, we design redox-active electrosorbents for the separation of PGM chloroanions, by leveraging the auto-oxidation of redox-electrodes and show that the energy consumption can be saved by 75% compared with standard electrosorption.
In this work, we applied four different metallopolymers with tunable redox-potentials and demonstrated their molecular selectivity in multicomponent PGM mixtures. Results showed that lower redox potential of the metallopolymer had higher uptake at OCP. Furthermore, the redox potential of ferrocene polymers was found to affect the selectivity towards PGMs ions, with a high molecular selectivity achieved of over 100 between Pt and Rh.
We further used a porous-coated redox-polymer electrosorbent for recovery of a leaching real-world catalytic converter with 186 mmol Pd uptake per mole of ferrocene moiety. Here, we provide a fully electrochemical and high energy-efficiency process-intensified platform for the multicomponent recovery of PGMs from waste feedstocks.