Adsorption and Diffusion of Oxygen on Pure and Partially Oxidized Metal Surfaces in Ultrahigh Resolution
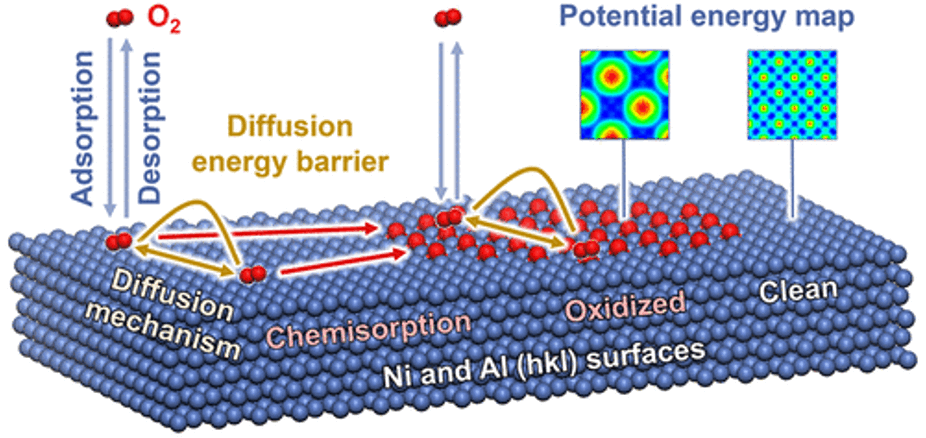
The interaction of gas molecules with metal and oxide surfaces plays a critical role in corrosion, catalysis, sensing, and heterogeneous materials. However, insights into the dynamics of O2 from picoseconds to microseconds have remained unavailable to date. 3D potential energy surfaces were obtained for adsorption of O2 on 11 common pristine and partially oxidized (hkl) surfaces of Ni and Al in picometer resolution and high accuracy of 0.1 kcal/mol, identified binding sites, and surface mobility from 25 to 300 °C. Relative oxidation rates and parameters for oxide growth were explained. Over 150,000 molecular mechanics and molecular dynamics simulations were employed with the interface force field (IFF) using structural data from X-ray diffraction (XRD) and low-energy electron diffraction (LEED). The methods reach 10 to 50 times higher accuracy than possible before and are suited to analyze gas interactions with metals up to the micrometer scale including defects and irregular nanostructures.