Monitoring the 2D Assembly of Peptides
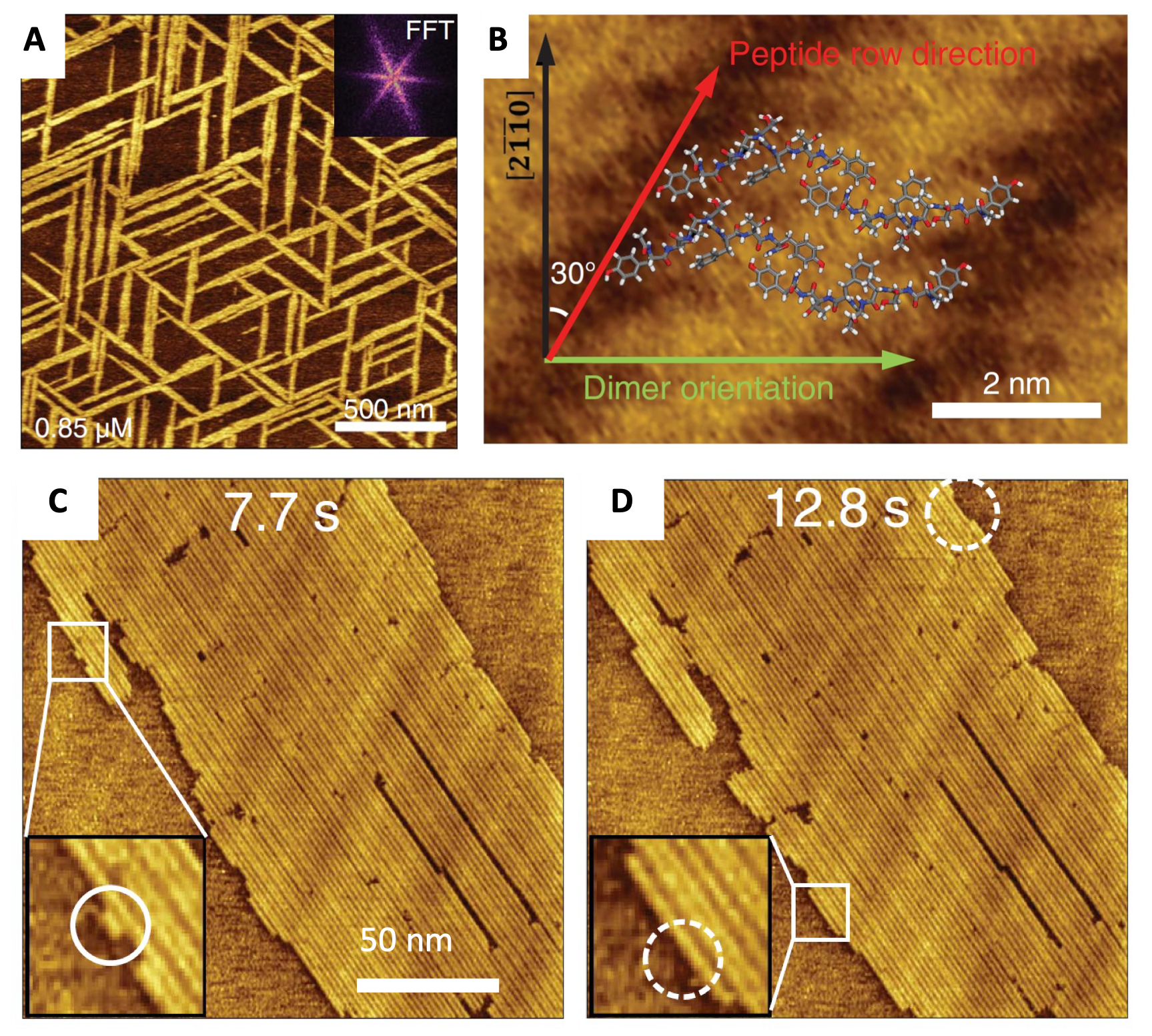
We identified the mechanism of nucleation and assembly of peptides on 2D layered substrates using a combination of biomimetic synthesis, highly resolved in-situ atomic force microscopy (AFM), and all-atom molecular dynamics simulations.
The time-resolved AFM imaging data could be resolved by simulations to supply the full atomic positions and quantitative energy data. Barrier-free nucleation in of atomic rows in 1D could be demonstrated whereby newly depositing peptides on the surface of molybdenum disulfide built up row-by-row and grow directionally highly ordered structures without an energy barrier. The results verify long-standing but unproven predictions of classical nucleation theory in one dimension while revealing key interactions underlying 2D assembly.
The study could provide engineers new design rules for creating microelectronics, membranes, and tissues, and open up better production methods for new materials.