Quantitative Insights into O2 Binding to Pt-M Surfaces
We introduced polarizable potentials for metals to accurately reproduce induced charges upon an applied voltage, complete surface/water interfacial properties, and binding of ligands on the 1 to 1000 nm scale. The calculations are a million times faster and partly more accurate than DFT methods (manuscript under review in Nat. Comm.).
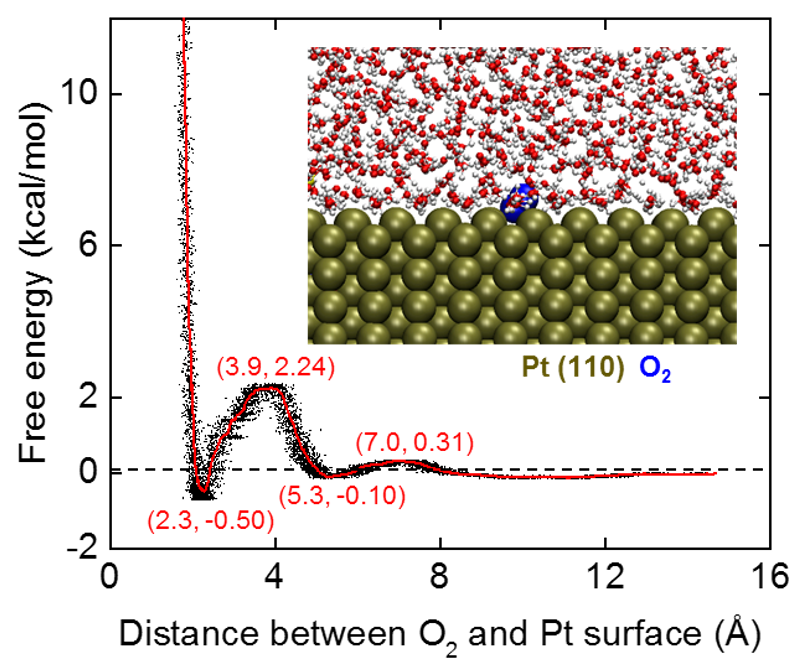
We applied these new MD tools to study the oxygen affinity to various metal and alloy facets. We found interesting correlations, such as preferred O2 adsorption on Pt (110) facets, medium adsorption on (111) facets, and inhibition of adsorption on (100) facets, which correlate with experimental activity measurements by Huang (see Figure).
The methods allow us to analyze oxygen binding energies on several alloy surfaces, nanowires, and shapes under realistic solution conditions for the first time. We have begun to derive revised volcano plots under various voltages, which could suggest direct principles for ORR catalyst design at a realistic scale.